A close friend, of mine, a clinical psychologist, asked me why different COVID- 19 vaccines were stored at different temperatures. As a neurologist, I didn’t know, but I promised her I would look it up and report back. Here is what I found. In Western Europe and North America, there are five major vaccines in the mix. 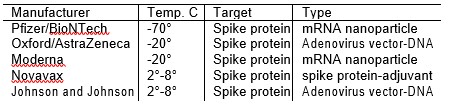
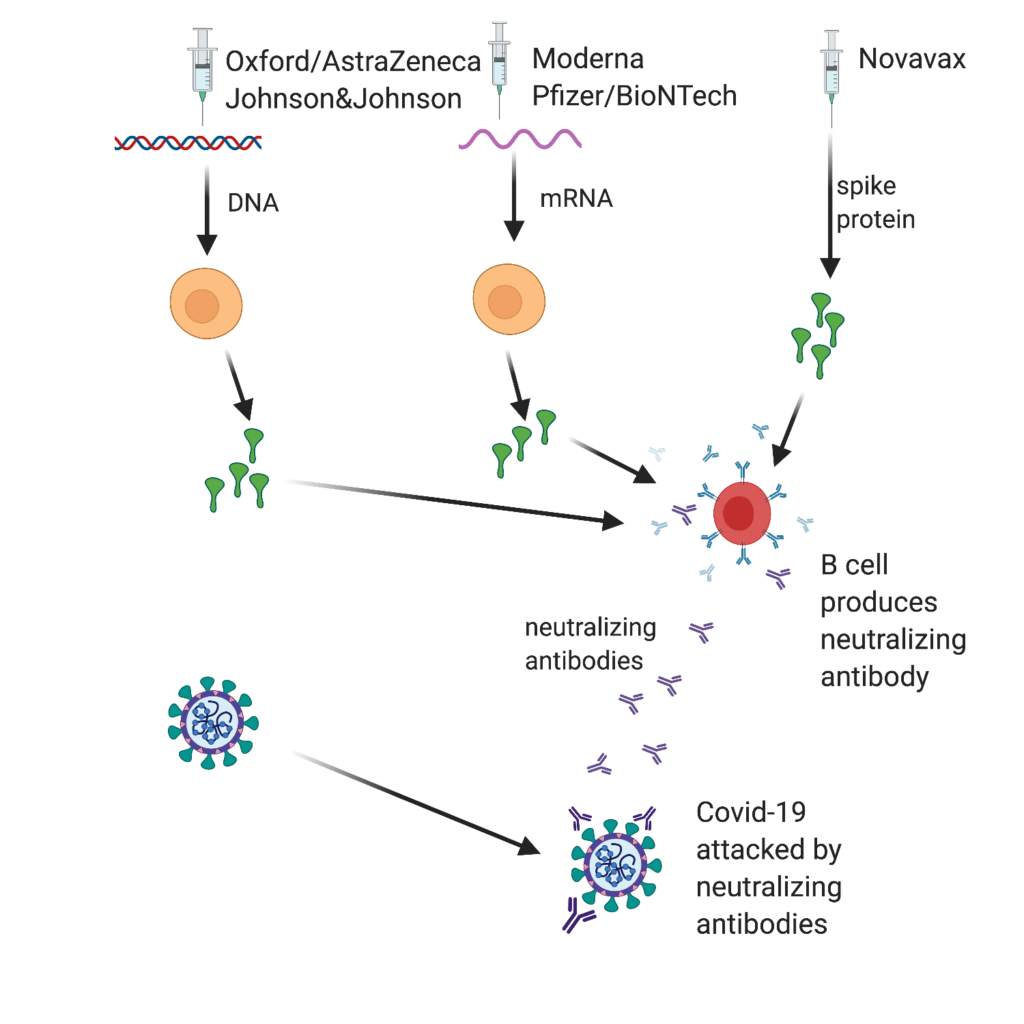
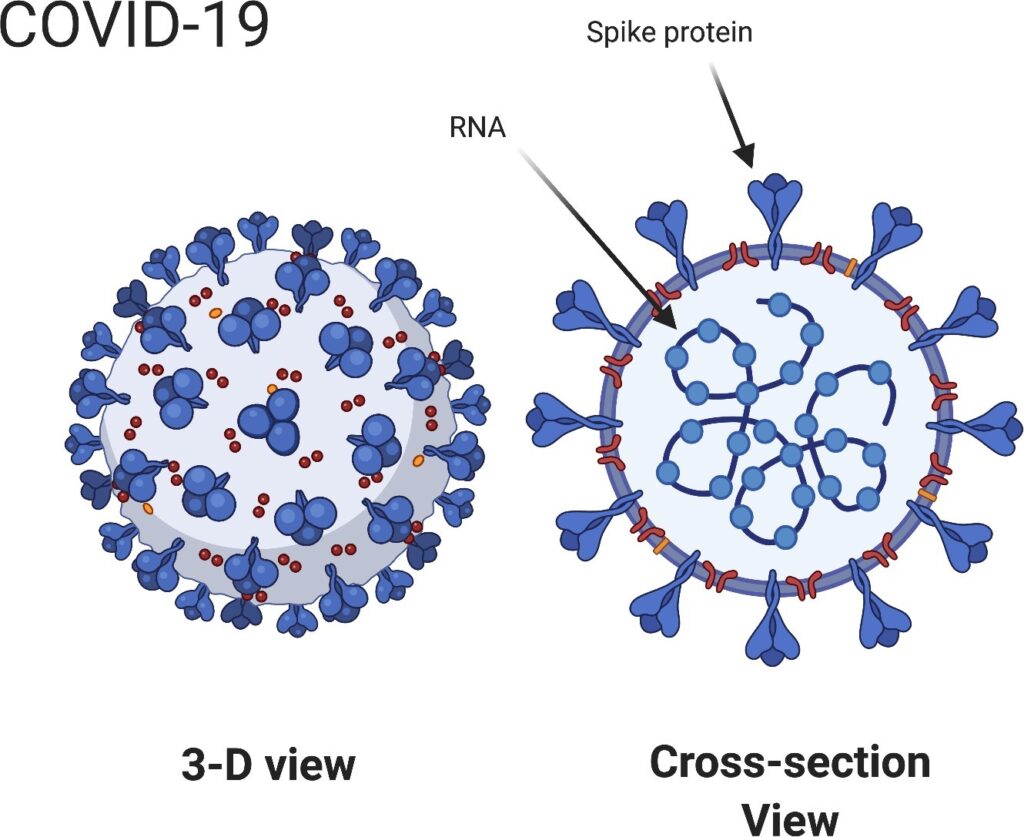 All of the vaccines target the spike protein on the COVID-19 virus (Figure 1). The goal of all five vaccines is to induce the body’s immune system to produce ”neutralizing” antibodies that prevent virus particles from adhering to cells, inserting their RNA, and initiating infections [1]. The effectiveness of each vaccine can be measured in the same way—how well does the vaccine do in inducing the body’s immune system to produce neutralizing antibodies against the COVID-19 virus?
All of the vaccines target the spike protein on the COVID-19 virus (Figure 1). The goal of all five vaccines is to induce the body’s immune system to produce ”neutralizing” antibodies that prevent virus particles from adhering to cells, inserting their RNA, and initiating infections [1]. The effectiveness of each vaccine can be measured in the same way—how well does the vaccine do in inducing the body’s immune system to produce neutralizing antibodies against the COVID-19 virus?
The amino acid sequence needed to make the spike protein can be coded as either RNA or DNA. The Johnson and Johnson vaccine codes the spike protein as a short strand of DNA and then introduces it into our bodies using a harmless adenovirus to deliver it to our cells. The Oxford-AstraZeneca vaccine works the same way. Once the DNA is in our cells, it triggers our cells to produce spike protein, which then triggers B cells (our antibody-making cells) to make neutralizing antibodies against the spike protein. Similarly, the mRNA vaccines, one from Moderna and one from Pfizer/BioNTech [2], deliver mRNA to cells in our bodies, which triggers the synthesis of spike proteins. To protect the mRNA from degradation so that it can reach the protein-synthesizing ribosomes within our cells, the mRNA is encased in a protective lipid nanoparticle. Cold temperatures are needed to stabilize these lipid nanoparticles until they are ready for injection at the time of vaccination. The mRNA triggers the synthesis of spike protein in injected muscle cells. When released, B cells react to the newly synthesized spike proteins and produce protective neutralizing antibodies. The Novavax vaccine is based on the direct injection of spike proteins (genetically engineered and synthesized in vitro) combined with an adjuvant to stimulate B cells to make neutralizing antibodies directly.
So why the difference in shipping and storage temperatures? The Novavax vaccine is based on the spike protein itself. It is stable at 2° to 8° C. The two vaccines which use adenoviruses as a vector to deliver DNA are also stable at 2° to 8° C. The two vaccines that deliver mRNA and depend on the lipid nanoparticles are less stable due to the fragile nature of the lipid nanoparticles that encapsulate the mRNA. If the lipid nanoparticles disintegrate, the vaccine becomes ineffective. Hence a lower storage temperature is needed to keep the nanoparticles stable [3, 4]
References
- Shibo Jiang, Christopher Hillyer, and Lanying “Neutralizing antibodies against SARSCoV-2 and other human coronaviruses”. In: Trends in immunol- ogy 41.5 (2020), pp. 355–359.
- Jonathan Corum and Carl Zimmer. How the Johnson and Johnson Vaccine Works. https://www.nytimes.com/interactive/2020/health/johnson-johnson-covid- 19-vaccine.html. Accessed: 2021-02-02.
- Carol Why Do COVID-19 Vaccines Have To Be Stored at Different Tem- peratures? //www.verywellhealth.com/covid-19-vaccine-temperature-storage- requirement-5091841. Accessed: 2021-02-02.
- Alex Phillippidis. ”The cold truth about COVID-19 vaccines”. https://www.genengnews.com/news/the- cold-truth-about-covid-19-vaccines/. ”Accessed: 2021-02-02”.
Figure 1: Vaccines work by inducing B cells (shown in red) to produce neutralizing antibodies to spike protein. The Oxford-AstraZeneca and the Johnson and Johnson vaccine use an adenovirus to deliver DNA that codes for spike protein. The Moderna and Pfizer-BioNTech vaccines deliver mRNA that codes for spike protein via lipid nanoparticles. Injected muscle cells (shown in yellow) and other cells can make spike protein. The Novavax vaccine delivers spike protein directly on injection combined with an immunological adjuvant. [Created by BioRender.com]